Reference Electrodes
Reference Electrodes: Overview
This Topic covers sub-topics such as Standard Hydrogen Electrode, Calomel Electrode and, Reference Electrodes
Important Questions on Reference Electrodes
A standard hydrogen electrode has zero electrode potential because
Calomel electrode is used as reference electrode.
Standard hydrogen electrode (SHE) is a
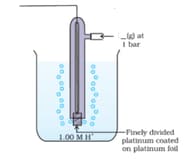
The IUPAC name of the gas released in the above diagram, where a blank is given is :
(Note : Fill the blanks by writing the chemical formulas in straightforward manner without using subscript. For example, the chemical formula of methane should be filled in the blank as and not as )
A reference electrode refers to an electrode that has a variable electrode potential.
The electrode potential of is fixed as
What is the reference electrode in determining the standard electrode potential?
Describe the construction and working of Normal Hydrogen Electrode?
The standard hydrogen electrode has zero electrode potential because
During the course of reaction, a half cell is connected with SHE, pH of SHE is increased. The connected half cell behaves as:
What would be of hydrogen electrode having ?
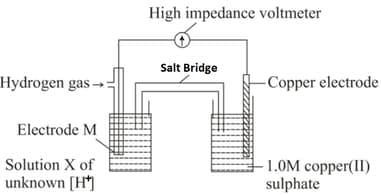
What could be the best material for electrode , if a student set up the following apparatus to determine the hydrogen ion concentration of solution X.
The cell is .
The reference electrode is made front which of the following?
Give the construction of NHE. What is its standard reduction potential? How does it help to determine the value of standard reduction potentials of other electrodes?
Match the list-I with List-II
List-I (Electrode) |
List-II (Type) |
||
|---|---|---|---|
Calomel |
Reference |
||
Glass |
Redox |
||
Hydrogen |
Membrane |
||
Quinhydrone |
Gas |
A standard hydrogen electrode has a zero potential because
Consider the cell
Given:
Calculate the for